Abstract
Leukemic stem cells have long been considered a therapy-resistant population in myeloid leukemia. Several recent publications have also described a population with stem/progenitor-like phenotype in pediatric B-cell acute lymphoblastic leukemia (B-ALL). However, the existence and cell-of-origin of stem/progenitor-like blasts in other subtypes of leukemias have not been studied extensively. Moreover, the relationship of this subpopulation with clinical outcomes and strategies to target them remain to be systematically explored in pediatric leukemia.
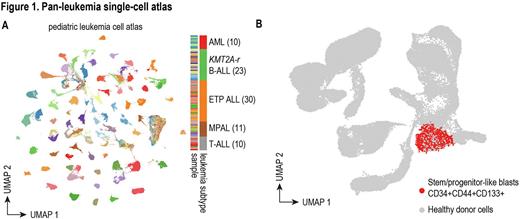
To investigate these questions, we extensively characterized diagnostic samples of 84 patients with high-risk pediatric leukemias, including AML (10), B-ALL (23), ETP T-ALL (30), non-ETP T-ALL (10), and mixed phenotype acute leukemia (MPAL) (11). We performed scRNA-Seq and scATAC-Seq on all 84 patient samples, generating an atlas of more than one million cells (Figure 1A, UMAP colored by sample). By projecting leukemic cells onto a novel healthy reference atlas composed of pediatric and infant bone marrow and thymus, we identified and characterized a stem/progenitor-like subpopulation with a common immunophenotype CD34+CD44+CD133+ in all five leukemia subtypes (Figure 1B). More importantly, we found the frequency of this subpopulation is highly correlated with the level of minimal residual disease after induction chemotherapy, suggesting therapy resistance of stem/progenitor-like leukemic blasts. We identified several pathways commonly up-regulated in stem/progenitor-like subpopulations across different high-risk leukemias, including previously reported leukemia stem cell (LSC) signatures in AML, quiescent signature, and ribosome biogenesis. To better power analyses relating stem/progenitor-like subpopulations with clinical outcome, we deconvoluted and analyzed >3000 bulk RNA-Seq samples from the TARGET and Children's Oncology Group AALL0434 T-ALL databases using our single-cell molecular signatures and found a universal existence of stem/progenitor-like leukemic blasts that predict poor clinical outcome across different high-risk leukemias.
Our single-cell analysis consistently associated chemoresistance with presence of stem/progenitor-like leukemic blasts across 5 subtypes of high-risk pediatric leukemia, leading us to seek novel therapeutic strategies targeting this subpopulation. Using single-cell derived cell signatures, we performed an in silico drug screen using multiple independent drug/target databases. This analysis revealed several pan-leukemia candidate drug targets for stem/progenitor-like leukemic blasts, including FLT3, CD44, CD133, BCL2, and PIK3R1. We also found leukemia-subtype-specific candidates such as BRAF for AML, TGFB1 for B-ALL, IL1B for ETP-ALL, and STAT3 for AML and B-ALL. Both pharmacological and genetic experiments using cell lines and PDX models are underway to validate the candidate drug targets.
Finally, we employed ligand-receptor analysis to identify interactions that stem/progenitor-like blasts could have with their surrounding environment. This analysis revealed a set of immunosupressive ligand-receptor pairs between the stem/progenitor-like leukemic blasts and NK cells. Using our predicted ligand-receptor ineractions, we computed patient-specific NK cytotoxic scores and discovered an immunosuppressive role of stem/progenitor-like leukemic blasts towards NK cells in all leukemia subtypes, suggesting a mechanism for immune surveillance escape and potential resistance to immunotherapy in stem/progenitor-like blasts.
Taken together, our data reveal the existence of a common stem/progenitor-like blast population in high-risk pediatric leukemias. These cells exhibit characteristics of leukemia stem cells, quiescence signatures, resistance to chemotherapy, and immunosuppressive effect towards NK cells. They are also highly predictive of poor clinical outcomes across different high-risk leukemias. We identified common and leukemia-subtype-specific candidate drug targets. Follow-up studies targeting these genes along with conventional chemotherapy are underway with promise to improve the clinical outcome for high-risk pediatric leukemias.
CC & JX, as well as DTT & KT, contributed equally to the work.
Disclosures
Raetz:Pfizer: Research Funding; BMS: Other: Data and Safety Monitoring Board. Hunger:Servier: Honoraria; Jazz: Honoraria; Amgen: Current equity holder in private company, Honoraria. Yang:Takeda: Research Funding. Mullighan:Illumina: Honoraria; Pfizer: Research Funding; Amgen: Honoraria; Abbvie: Research Funding; BEAM: Honoraria; Consulting: Honoraria; FAZE: Honoraria. Bernt:Merck: Other: Husband is an employee of Merck and has stock; Syndax: Research Funding; Epizyme: Patents & Royalties. Teachey:Sobi: Consultancy; BEAM Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal